The little, juvenile and aggressive “fluorine atom” is growing up
Author(s): Navarrini, Walter.
Source: CHIMICA OGGI-CHEMISTRY TODAY Volume: 28 Issue: 2 Pages: 2-2 Published: MAR-APR 2010
Author(s): Navarrini, Walter.
Source: CHIMICA OGGI-CHEMISTRY TODAY Volume: 28 Issue: 2 Pages: 2-2 Published: MAR-APR 2010
Author(s): Bianchi, Claudia L.; Ardizzone, Silvia; Cappelletti, Giuseppe; Cerrato, Giuseppina; Navarrini, Walter; Sansotera, Maurizio.
Source: JOURNAL OF MATERIALS RESEARCH
Volume: 25 Issue: 1 Special Issue: SI Pages: 96-103
DOI: 10.1557/JMR.2010.0008 Published: JAN 2010
ABSTRACT:
A high-molecular-weight perfluoropolyether (PFPE-YR) and a perfluoropolyether containing ammonium phosphate (PFPE-F10) have been evaluated as fluorinated coating for high-surface-area titanium oxides. Coated nano-TiO2 shows hydrophobic properties and excellent buoyancy on water. In addition to photoactivity toward the degradation of toluene in gas phase, specific trial analyses have been completed to estimate the modified titanium oxide features. Brunauer-Emmett-Teller (BET) analysis for the surface area determination, ultraviolet-visible spectroscopy (UV-Vis) for the material electronic band gap, high-resolution transmission electron microscopy (HRTEM), x-ray diffraction (XRD), and x-ray photoelectron spectroscopy (XPS) for the morphology, structure, and surface composition, respectively, and water contact angle and infrared (IR) analysis have been performed to estimate the wettability and stability of coated titanium.
http://journals.cambridge.org/action/displayAbstract?fromPage=online&aid=7938175
 Author(s): Sansotera, Maurizio; Bianchi, Claudia L.; Lecardi, Giorgio; Marchionni, Giuseppe; Metrangolo, Pierangelo; Resnati, Giuseppe; Navarrini, Walter.
Author(s): Sansotera, Maurizio; Bianchi, Claudia L.; Lecardi, Giorgio; Marchionni, Giuseppe; Metrangolo, Pierangelo; Resnati, Giuseppe; Navarrini, Walter.
Source: CHEMISTRY OF MATERIALS
Volume: 21 Issue: 19 Pages: 4498-4504
DOI: 10.1021/cm901271q
Published: OCT 13 2009
ABSTRACT:
Perfluorocarbon chains, that is, perfluoroethyl, CF(3)CF(2)-, pernuoro-n-propyl, CF(3)CF(2)CF(2)-, perfluoro-iso-propyl, (CF(3))(2)CF-, and perfluoropolyether (PFPE) residues, were covalently bonded on the surface of a carbon black using perfluorodiacyl and PFPE peroxides. Thermal decomposition of the fluorinated peroxides allows the covalent linkage of fluorinated radicals to the polycyclic aromatic structure of the carbon black. Contact angle measurements in agreement with XPS data revealed a gradual enhancement of the water repellence with increasing fluorine content on the surface. When perfluoroalkyl chains were bonded to the carbon black the morphology and the surface areas of the modified carbonaceous materials were mostly retained as shown by BET and SEM analyses.
http://pubs.acs.org/doi/abs/10.1021/cm901271q
Author(s): Avataneo, M.; Navarrini, W.; De Patto, U.; Marchionni, G.
Source: JOURNAL OF FLUORINE CHEMISTRY
Volume: 130 Issue: 10 Pages: 933-937
DOI: 10.1016/j.jfluchem.2009.07.007 Published: OCT 2009
ABSTRACT:
Peroxidic perfluoropolyethers (PFPEs) are industrial intermediates used by Solvay Solexis for the preparation of different classes of (per)fluoropolyethers (Fomblin (R), Galden (R), Solvera (R), Fluorolink (R)). The chemistry of these peroxidic compounds has been recently exploited for the synthesis of novel PFPE block copolymers. In the present work we report the synthesis, the structural and physical-chemical characterization of block copolymers obtained by the reaction of peroxidic PFPEs with 2,2,4-trifluoro-5-trifluoromethoxy-1,3-dioxole, a cyclic homopolymerizable perfluoroolefin. These block copolymers combine the most attractive properties of the PFPEs, like the excellent lubrication, the high thermal stability and the optical transparency, with new specific properties which are related to the perfluorodioxolenic blocks.
http://www.sciencedirect.com/science/article/pii/S0022113909002036
M. Sansotera a, W. Navarrini a, P. Metrangolo a, G. Resnati a, C. Bianchi b, A. Guarda c
a Dip-CMIC ”Giulio Natta”, Politecnico di Milano, via Mancinelli 7, 20131 Milan, Italy;
b Department of Physical Chemistry and Electrochemistry, University of Milan, via Golgi 19, 20133, Milan, Italy
c R&D Centre, Solvay Solexis, viale Lombardia 20, 20021 Bollate (MI), Italy
E-mail: maurizio.sansotera@polimi.it
Perfluorodiacyl peroxides and perfluoropolyether peroxides (FOMBLIN peroxides) are suitable organic peroxides for direct linkage of perfluoroalkyl and perfluoropolyether chains on unsaturated substrates through a radical pathway. Highly graphitic carbon black and a-C:H diamond-like carbon are carbonaceous materials characterized by sp2 hybridized carbon atoms in the structure. Consequently a chemical treatment with high fluorine content organic peroxides allows the introduction of fluorinated groups onto carbonaceous substrates with carbon-carbon bond formation [1]. Depending on the peroxide involved during the treatment, several fluorinated chains, i.e. perfluoroethyl, CF3CF2-, perfluoro-n-propyl, CF3CF2CF2-, perfluoro-iso-propyl, (CF3)2CF-, and perfluoropolyether residual, has been covalently linked on the surface of such carbonaceous materials. Functionalization of carbon black transfers the typical hydrophobic properties of perfluorinated chains to the carbon black surface even preserving its conductive properties. This chemical treatment on diamond-like carbon films produces a covalently linked protective layers with the typical lubricant properties of perfluorinated compounds.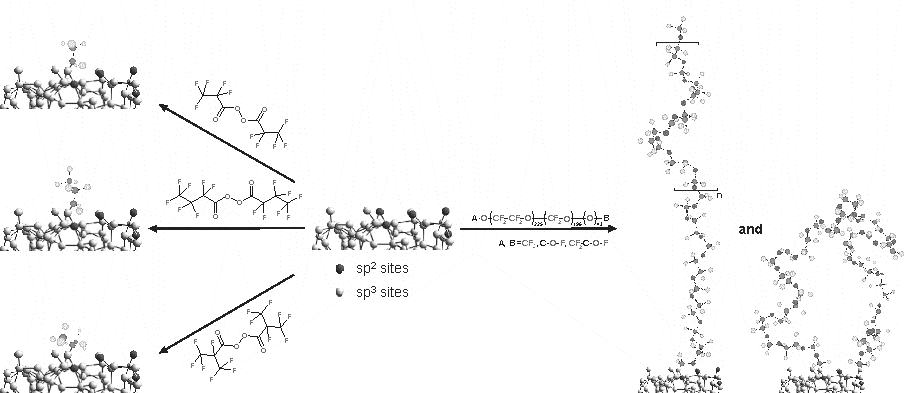
[1] Navarrini W., Sansotera M., Metrangolo P., Cavallotti P., Resnati G. WO 2009/019243 A1.
 Author(s): SANSOTERA M., VENTURINI F., BERETTA S., BASSI M., PASQUALI A., BIANCHI C. L., METRANGOLO P., RESNATI G., NAVARRINI W.
Author(s): SANSOTERA M., VENTURINI F., BERETTA S., BASSI M., PASQUALI A., BIANCHI C. L., METRANGOLO P., RESNATI G., NAVARRINI W.
Source: Chimica Oggi – Chemistry Today.
Volume: 27 Pages: 56-59.
Published: Jun. (2009).
ABSTRACT:
Perfluorodiacyl peroxides are suitable tools for thecovalent linkage of fluorinated groups on substrates containing aromatic moieties. The thermal decomposition process of such fluorinated peroxides allows the covalent linkage of fluorinated radicals on the polycyclic aromatic structure of a graphitic carbon black. Depending on the perfluorodiacyl peroxide involved during the chemical treatment, several fluorinated chains, i.e. perfluoroethyl, CF3CF2-, perfluoro-n-propyl, CF3CF2CF2- and perfluoro- iso-propyl, (CF3)2CF-residues have been bonded covalently on the surface of a conductive carbon black. At the characteristic temperature of perfluorocarbons thermolysis (400 C), thermogravimetric analyses revealed weight loss in agreement with the fluorine contents on the modified carbon blacks.
US Patent: 7534845 B2
Date: May 19, 2009
Inventor: W. Navarrini
Navarrini Walter a*, Venturini Francesco a, Sansotera Maurizio a, Metrangolo Pierangelo a, Resnati Giuseppe a, Galimberti Marco b, Barchiesi Emma b, Dardani Patrizia b .
a Dipartimento di Chimica, Materiali e Ingegneria Chimica, Politecnico di Milano, 7, via Mancinelli, I-20131, Milano, Italy.
b Solvay-Solexis, R &D Centre, 20, viale Lombardia, I-20021 Bollate (MI), Italy.
In the reaction between perfluoroolefins and perfluoroalkylhypofluorites [1] the existence of two different free radical reaction mechanisms is demonstrated by the presence of characteristic by-products.
In particular in the reaction between trifluoromethyl hypofluorite and highly reactive perfluoroolefins like CF2=CFOCF3 and CF2=CF2, the free radical oligomerization and dimerization products can be suppressed by utilizing the opportune experimental conditions.
These experimental conditions are characterized by the presence of hypofluorite during the addition reaction and can be performed by adding the olefin to the hypofluorite, these conditions herein referred as ”Reverse hypofluorite addition” are different from the standard methodologies described in the literature where generally the hypofluorite is added to the olefin.
The main products of the addition of CF3OF to CF2=CFOCF3 are perfluoro-2,2-bis-(methoxy)-ethane and perfluoro-1,3-bis-(methoxy)-ethane [2] in the molar ratio of 20% and 80% respectively.
We found experimental evidence for the termination products peroxide CF3OOCF3 and perfluoroethersC8F18O4respectively. The first termination product CF3OOCF3 was present at high amount in the reaction where the CF3OF concentration was maintained always above zero and substantially constant, on the contrary the termination products C8F18O4 were completely absent. In this conditions the oligomerization and polymerization of the perfluoroolefins were also suppressed, as well as in the case of the very reactive TFE.
[1] W. Navarrini, V. Tortelli, A. Russo, S. Corti, J. Fluorine Chem. 95(2),(1999)27-39.
[2] W. Navarrini, G. Resnati, P. Metrangolo, M. Cantini, F. Venturini, IT Patent app. MI2007A001384 (2007).
 Author(s): Navarrini, Walter; Venturini, Francesco; Sansotera, Maurizio; Metrangolo, Pierangelo; Resnati, Giuseppe; Galimberti, Marco; Tortelli, Vito.
Author(s): Navarrini, Walter; Venturini, Francesco; Sansotera, Maurizio; Metrangolo, Pierangelo; Resnati, Giuseppe; Galimberti, Marco; Tortelli, Vito.
Source: ABSTRACTS OF PAPERS OF THE AMERICAN CHEMICAL SOCIETY
Volume: 236
Meeting Abstract: 34-FLUO Published: AUG 17 2008
ABSTRACT:
The addiction reaction of hypofluorite to halogenated olefins has been studied in the past. In our previous studies we have noticed that the concentration of hypofluorite in the reaction media is an important variable to control the course of the main reaction. This experimental approach allowed the identification of a straight and economical methodology for the preparation of highly pure perfluoroethers with low boiling point and low Ostwald coefficient. These perfluoroethers have been recently tested as fluids for the preparation of injectable microbubble compositions as contrast agents in diagnostic ultrasound imaging. In many ultrasounds imaging applications there is the need for more efficient contrast agents and efforts to develop suitable materials are ongoing.
 Author(s): VENTURINI F., METRANGOLO P., RESNATI G., NAVARRINI W., TORTELLI V.
Author(s): VENTURINI F., METRANGOLO P., RESNATI G., NAVARRINI W., TORTELLI V.
Source: Chimica Oggi – Chemistry Today.
Volume: 26 Pages: 36-38. Published: Aug. (2008).
ABSTRACT:
The products deriving from the addition of CF2=CFOCF3 to CF3OF are proved to be low Ostwald coefficient fluids suitable for producing high stable micro bubbles that can be used as ultrasound imaging contrast agents. The standard gas-liquid fast radical chain reaction between trifluoromethyl hypofluorite CF3OF and reactive perfluoroolefins like CF2=CFOCF3produces oligomerization and dimerization byproducts. Therefore a purification procedure is needed in order to separate the low Ostwald coefficient products from the reaction mixture. Surprisingly we have found that the selectivity of the reaction can be increased by modifying the experimental reaction conditions adopted. This behaviour is an experimental evidence for the presence of two different reaction schemes that can be switched by tuning the hypofluorite concentration in the reaction media.
 Author(s): NAVARRINI W., VENTURINI F., SANSOTERA M., URSINI M., METRANGOLO P., RESNATI G., GALIMBERTI M., BARCHIESI E., DARDANI P.
Author(s): NAVARRINI W., VENTURINI F., SANSOTERA M., URSINI M., METRANGOLO P., RESNATI G., GALIMBERTI M., BARCHIESI E., DARDANI P.
Source: Journal of Fluorine Chemistry.
Volume: 129 Issue: 8 Pages: 680-685.
DOI: 10.1016/j.jfluchem.2008.05.018. Published: Aug. (2008).
ABSTRACT:
In the reaction between perfluoroolefins and perfluoroalkylhypofluorites the existence of two different free radical reaction mechanisms is demonstrated by the presence of characteristic byproducts. These kinetic schemes can be experimentally controlled by tuning the hypofluorite concentration in the reaction media. In particular, in the reactions between trifluoromethyl hypofluorite and highly reactive perfluoroolefins like CF2CFOCF3 and CF2CF2, the free radical oligomerization and dimerization products can be suppressed by utilizing the opportune experimental conditions. The pure perfluorinated ethers obtained, having low Ostwald coefficient, can be utilized as contrast agents for diagnostic ultrasound imaging injectable microbubbles composition.
http://www.sciencedirect.com/science/article/pii/S0022113908001486