Peroxidic perfluoropolyether for the covalent binding of perfluoropolyether chains on carbon black surface.
 Author(s): SANSOTERA M., NAVARRINI W., GOLA M., BIANCHI C. L., WORMALD P., FAMULARI A., AVATANEO M.
Author(s): SANSOTERA M., NAVARRINI W., GOLA M., BIANCHI C. L., WORMALD P., FAMULARI A., AVATANEO M.
Source: Journal of Fluorine Chemistry.
Volume: 132 (12) Pages: 1254-1261.
DOI: 10.1016/j.jfluchem.2011.07.018. Published: Dec. (2011).
ABSTRACT
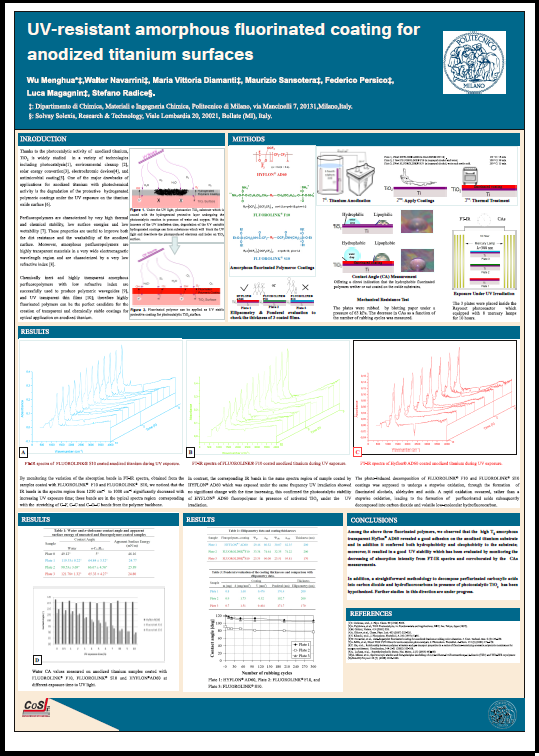
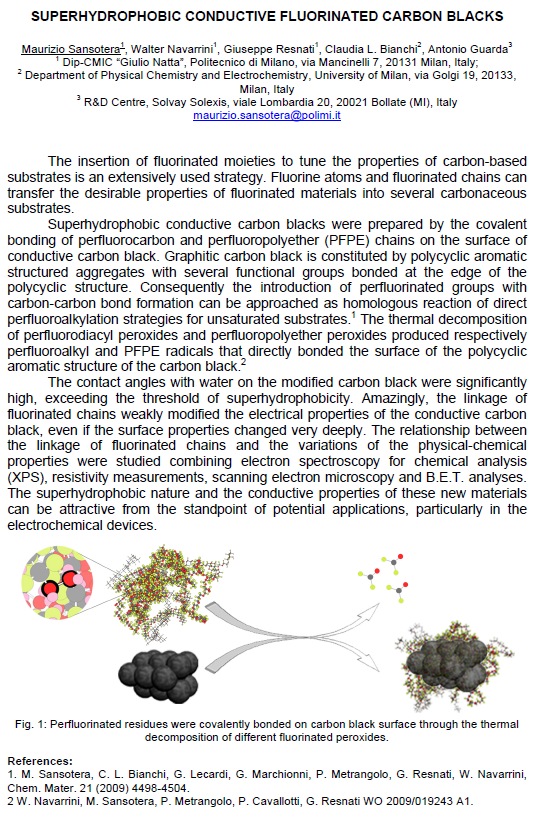
Peroxidic perfluoropolyethers (PFPEs) are suitable tools for the covalent linkage of fluorinated groups on substrates containing aromatic moieties. Thus the thermal decomposition process of such fluorinated peroxides allowed the covalent linkage of PFPE radicals to the polycyclic aromatic structure of a graphitic carbon black. Contact angle measurements on molded pellets made with modified carbon black powders revealed a gradual enhancement of the hydrophobicity, which follows the increase of the fluorine content on the surface according to XPS experiments. BET analyses also revealed variations of the surface area of carbonaceous samples. Products and by-products were also evaluated by mass balances of decomposed portions of PFPE residues, respectively, PFPE chains bonded on carbon black and PFPE fluids obtained by homocoupling side-reactions. Modified carbonaceous materials were analyzed by solid state 19F-MAS NMR and the results are in agreement with the proposed radical mechanism.
http://www.sciencedirect.com/science/article/pii/S0022113911002831










 Author(s): NAVARRINI W., SANSOTERA M., VENTURINI F., BIANCHI C. L., GUARDA P. A., RESNATI G.
Author(s): NAVARRINI W., SANSOTERA M., VENTURINI F., BIANCHI C. L., GUARDA P. A., RESNATI G.